Introduction
Pediatric acute myeloid leukemia (pedAML) is a heterogeneous group of hematological malignancies classified according to morphology, immunophenotyping, and genomics. The optimal adjustment of therapy based on level of response is not well defined.
Aim:
Herein, we present the results of a retrospective study aiming to analyze the prognostic value of measurable residual disease (MRD) as determined by flow cytometry (FC-MRD) in pediatric AML.
Patients and Methods
All pediatric and adolescent patients (aged 0-18 years old) with AML treated in our unit between 1/2013-6/2023 were included in this study. French American British (FAB) morphological classification, immune-phenotype analysis, and molecular characterization were performed on diagnosis, and all patients were treated according to AML-BFM protocols. Final risk classification was based on evaluation of response and on genetic abnormalities at diagnosis.
MRD was assessed by FC-MRD after Induction 1 and 2, and, either after course 5 or before hematopoietic stem cell transplantation (HSCT). Complete Remission (CR) was defined as regenerative or normocellular bone marrow with <5% blasts; in peripheral blood ≥1.0x10 9/L neutrophil granulocytes and ≥80x10 9/L thrombocytes or Remission with partial regeneration ( CRp: regenerative or normocellular bone marrow with <5% blasts; in the blood: ≥1.0x10 9/L leukocytes, ≥0.5x10 9/L, neutrophil granulocytes and ≥50x10 9/L, self-sustained platelets), lasting at least 4weeks; no extramedullary disease.
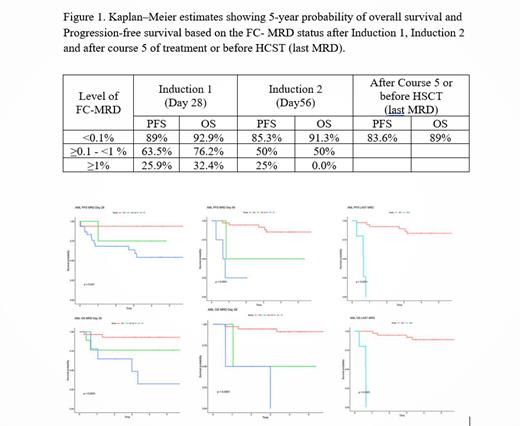
Overall survival (OS) was defined as the time interval from the treatment initiation date to the date of death due to any cause. Progressive free survival (PFS) was defined as the time interval from the response date to the date of disease relapse or death due to any cause. The probabilities of OS and PFS were estimated with the Kaplan-Meier method.
Results
Overall, there were 52 patients (median age at diagnosis:7 years, 37males/15 females) diagnosed with AML, of whom: 40 patients with de novo AML, 5 patients with therapy- or myelodysplasia-related AML and 7 patients with mixed-lineage leukemia.
Cytogenetics were considered favorable for 16 (31.0%), intermediate for 4 (7.6%), and adverse for 32 (61.4%) patients. Standard-risk patients (n=16) received only chemotherapy, whereas all high-risk patients, and intermediate-risk patients with available matched donors, underwent HSCT should they have reached remission. Forty-four patients (84.6%) achieved complete response (CR). Among these patients, 43 patients (97.7%) achieved CR after 1 course of induction, and 1 patient (2.3%) required at least 2 courses. Twenty-four patients (46.1%) underwent HSCT during either their first (20/24) or second (4/24) CR.
Overall, 12 patients (23.07%) died, 4 patients due to resistant disease, 3 patients due to relapse, 2 patients due to serious complications upon diagnosis, 1 patient due to infection and 2 patients due to transplant-related complications.
With a median follow-up time of 3.7 years (range 0.1-11.1years), the five-year OS and PFS of the entire cohort was 74.0% and 70.0%, respectively. The level of FC-MRD was a significant prognostic factor for OS and PFS. More specifically, achievement of FC-MRD < 0.1% at all time points was associated with a significant improvement in PFS and OS. (figure 1). FC-MRD ≥ 0.1% after course 5 or before HSCT was the only independent adverse prognostic factor that was statistically significant for both progression-free (P=0.001) and overall survival (P<0.001) (figure1).
Conclusions
We investigated the association between FC-MRD and survival in Greek pediatric patients with AML. In our cohort, achieving an MRD status <0.1% according to FC is associated with significant improvement in the outcome of pediatric patients with AML.
Disclosures
Kattamis:Agios Pharmaceuticals: Consultancy; Vertex Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Chiesi: Honoraria; Bristol Myers Squib/Celegene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Consultancy; Ionis Pharmaceuticals: Consultancy; Vifor: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal